Abstract
Background
In untreated FL, rituximab (R)-bendamustine leads to overall response (OR), complete response (CR), and 3-year progression-free survivals (PFS) of 91-97%, 31-40%, and 68-70%, respectively. The GALLIUM study demonstrated an improved PFS with obinutuzumab-chemotherapy compared to R-chemotherapy with 3-year PFS 80% and 73.3%, respectively. In relapsed FL, phase 2 trials demonstrated OR and CR of 88% and 53% with combined rituximab, bendamustine, and bortezomib. We conducted a multicenter, randomized phase 2 trial to determine the CR with ofatumumab and bendamustine and with ofatumumab, bendamustine, and bortezomib induction and maintenance in pts with untreated high risk FL.
Methods
Pts with untreated grade 1-3a FL and either a FL international prognostic index (FLIPI) of 2 with at least one lymph node > 6 cm or FLIPI 3-5 were enrolled. Pts were randomized to arm A (ofatumumab 1000 mg day (d) 1 with bendamustine 90 mg/m2 d 1 and 2 every 35 d for 6 cycles followed by maintenance ofatumumab 1000 mg d 1 every 2 months (mos) for 4 cycles) or to arm B (ofatumumab 1000 mg d 1, bendamustine 90 mg/m2 d 1 and 2, and bortezomib 1.6 mg/m2 (IV or SQ) d 1, 8, 15, and 22 every 35 d for 6 cycles followed by maintenance ofatumumab 1000 mg d 1 and bortezomib 1.6 mg/m2 d 1, 8, 15, and 22 every 2 mos for 4 cycles). Response was assessed by FDG PET/CT after cycles 2 and 6 of induction and by CT every 4 mos during maintenance and every 6 mos until progression. The primary endpoint was CR, targeting a CR of 40% in A and 60% in B. A 2-stage design was used to compare CR between treatment groups. Evaluation of PFS and toxicity were secondary endpoints.
Results
From 2011-2016, 135 pts enrolled with 127 (A=66 and B=61) eligible for response assessment. The median age was 61 (range 25-87), 73% had grade 1-2 FL, 33% were stage III, 65% were stage IV, 19% had B-symptoms, 47% had bulky disease > 6 cm, 29% had elevated LDH, 28% were FLIPI 2, and 69% FLIPI 3-5. In A, 58 (87%) pts completed induction and 38 (57%) completed maintenance. In B, 42 (65%) and 27 (42%) completed induction and maintenance. Eight pts in A and 9 in B are still receiving maintenance. Reasons for early termination included adverse events (A=12 and B=9), progression (A=2 and B=6), pt refusal (A=4 and B=7), death due to sepsis (B=1), and other (A=3 and B=6). Dose reductions or delays were required in 66% and 86% pts on A and B, respectively. Grade 3-4 events occurred in 75% A and 75% B. These events included neutropenia (A=31%, B=27%), thrombocytopenia (A=2%, B=5%), febrile neutropenia (A=1%, B=3%), infection (A=7%, B=12%) nausea/vomiting (A=0, B=8%), diarrhea (A=4%, B=9%), fatigue (A=4%, B=11%), infusion reactions or cytokine release (A=5%, B=10%), and peripheral neuropathy (A=0, B=3%).
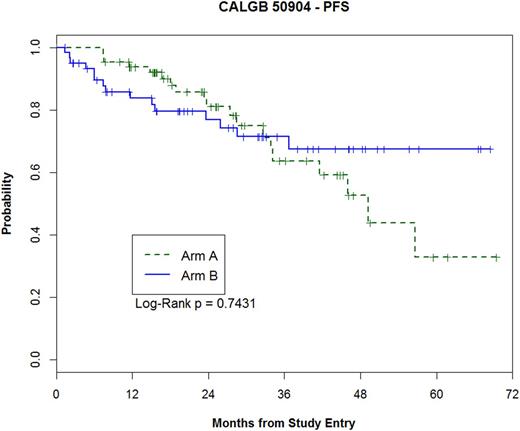
For A, the OR was 92% (n=61, 95% CI 83-97%) with 59% CR (95% CI 46-71%) and for B, the OR was 84% (n=51, 95% CI 72-92%) with 57% CR (95% CI, 44-70%), with no significant difference in CR in A or B (p=0.65). Thirty pts have progressed (A=18 and B=12). Ten pts have died (A=3 and B=7), due to FL (5), sepsis (1), lung cancer (1), and unknown cause (3). With a median follow-up of 25 mos in A (< 1-70 mos), the 2-, 3-, and 4-year PFS are 81%, 64%, and 53%. With a median follow-up of 22 mos in B (1-69 mos), the 2-, 3-, and 4-year PFS are 77%, 71%, and 67% (see figure). The 4-year overall survival in A and B are 87% and 84%, respectively. The surrogate endpoint EFS24 is 81% in A and 75% in B. In univariable analysis, elevated LDH was significantly associated with an inferior PFS (p=0.014), while FLIPI 2 vs. 3-5, stage, and bulk > 6 cm were not associated with OR, PFS, or OS.
Conclusion
In this multicenter randomized phase 2 trial, the OR, CR, and PFS with ofatumumab and bendamustine and with ofatumumab, bendamustine, and bortezomib are similar in pts with previously untreated high risk FL, with no significant difference with intensification of therapy with bortezomib. Although grade 3-4 toxicities are similar, more pts treated with bortezomib required dose modifications and early discontinuation. While not the primary endpoint of this trial, the addition of ofatumumab to bendamustine appears to improve CR when compared to historical data with R-bendamustine in untreated FL. However, 3-year PFS rates are similar to historical data and a randomized study would be required to determine if ofatumumab improves PFS as recently observed with obinutuzumab. Support: U10CA180821, U10CA180882. ClinicalTrials.gov Identifier: NCT01286272
Blum: Morphosys: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Research Funding; Novartis: Research Funding; Cephalon: Research Funding; Millenium: Research Funding; Pharmacylics: Research Funding; Janssen: Research Funding. Hsi: Eli Lilly: Research Funding; Abbvie: Research Funding; Cellerant Therapeutics: Research Funding; Seattle Genetics: Membership on an entity's Board of Directors or advisory committees. Wagner-Johnston: Novartis: Research Funding; Celgene: Research Funding; Gilead: Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Membership on an entity's Board of Directors or advisory committees; JUNO: Membership on an entity's Board of Directors or advisory committees; Regeneron: Research Funding. Cheson: Celgene: Consultancy; Roche-genentech: Consultancy, Research Funding; Pharmacyclics: Research Funding. Bartlett: Novartis: Research Funding; ImaginAB: Research Funding; Astra Zeneca: Research Funding; Millenium: Research Funding; Janssen: Research Funding; Pharmacyclics: Research Funding; Affimed: Research Funding; Forty Seven: Research Funding; Immune Design: Research Funding; Bristol-Meyers Squibb: Research Funding; Merck & Co: Research Funding; KITE: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech: Research Funding; Seattle Genetics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal